- Shanghai Zhongshen International Trade Co., Ltd. - Two decades of trade agency expertise.
- Service Hotline: 139 1787 2118
South KoreaMedical EquipmentThe market access process is relatively complex, involving multiple steps and the participation of multiple departments. This article will briefly introduce the certification and registration processes required for exporting medical devices to South Korea.
Competent Department
The Ministry of Food and Drug Safety (MFDS) of South Korea is the competent department for medical devices in South Korea, responsible for the safety of food, drugs, medical devices, the development of the food and pharmaceutical industries, and the promotion of public health.Cosmetics & Personal CareMedical Device Classification
South Korea classifies medical devices into four categories, namely Class I, II, III, and IV. This classification method is very similar to the EUs classification method for medical devices. Each category of devices has different market access channels, and the approval processes and time also vary based on the risk classification of medical devices.
Product Testing and Clinical Trials
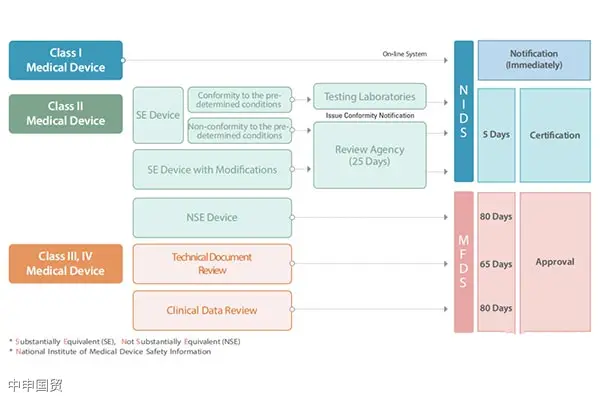
Product samples that need to be registered can be directly sent to South Korean laboratories for testing, or tested in domestic laboratories with corresponding South Korean qualifications, and a qualified test report shall be provided. MFDS has designated a series of testing laboratories and designated hospitals for clinical trials.
System Assessment
Website for applying for South Korean cosmetics certificates:
Manufacturers exporting Class II, III, and IV medical devices to South Korea must comply with KGMP (Korean Good Manufacturing Practice) requirements, which are similar to ISO 13485. The KGMP certificate is issued to importers rather than manufacturers and must be renewed every 3 years.
South Korean Medical Device Registration Processhttps://kcia.or.kr/cert/main/
The import of medical devices in South Korea is divided into the following steps: Select a South Korean license holder, product registration, document preparation, product testing, clinical trials, product registration certificate, KGMP document preparation, hospital access.
Product Launch and Post - launch Supervision
After the product registration certificate and KGMP certificate are issued, for non - household medical devices, hospital access is also required, entering the hospitals medical insurance system and obtaining a hospital medical insurance number.此后, the product can be officially sold in the South Korean market. After the product is launched, MFDS has the right to track some designated high - risk medical device products. Products with adverse reactions in the South Korean market should be recalled in a timely manner.
Finally, the conditions for foreign products to enter the South Korean market are: very detailed product technical documents and materials required for applying for South Korean KGMP need to be prepared; the companys system should meet the requirements of ISO 13485; the product should meet the standard requirements. The above is the relevant system for the access of medical devices in South Korea, hoping to be helpful to medical device enterprises.
Exporting Medical Devices to the Brazilian Market: Access Rules and Key Points of Regulationsimport and exportOrthokeratology Lenses: Inspection, Documentation and Regulatory Requirements
Related Recommendations
Category case
Contact Us
Email: service@sh-zhongshen.com
Related Recommendations
Contact via WeChat

? 2025. All Rights Reserved. Shanghai ICP No. 2023007705-2  PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912